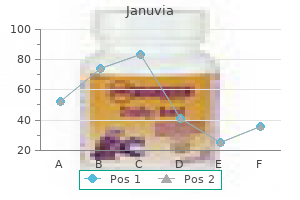
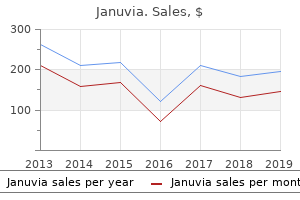
"Discount januvia on line, diabetes in bichon frise dogs".
By: Q. Porgan, M.A., Ph.D.
Clinical Director, University of Illinois College of Medicine
There is an organized fibrous exudate and the underlying endocardium is thickened by fibrous tissue diabetes type 2 interventions purchase januvia visa. Although there are large numbers of circulating eosinophils diabetes injectable medications list purchase januvia line, these cells are not seen in the injured endocardium diabetes symptoms new zealand januvia 100mg low cost, which is thought to be damaged by granules released from circulating eosinophils metabolic disease caused by accumulation of uric acid cheap januvia 100 mg online. The panel at the bottom shows two partly degranulated eosinophils (center) surrounded by erythrocytes in a peripheral blood film. A more widespread edematous response, as shown in the upper right panel, develops approximately 8 hours later and can persist for some hours. Similarly, the response to an inhaled antigen can be divided into early and late responses (bottom panel). The immediate response peaks within minutes after antigen inhalation and then subsides. The immediate response is caused by the direct effects on blood vessels and smooth muscle of rapidly metabolized mediators such as histamine released by mast cells. Eosinophils and basophils cause inflammation and tissue damage in allergic reactions. Their continued presence is characteristic of chronic allergic inflammation and they are thought to be major contributors to tissue damage. There is evidence for reciprocal control of the maturation of the stem-cell population into basophils or eosinophils. Basophils are normally present in very low numbers in the circulation and seem to have a similar role to eosinophils in defense against pathogens. Eosinophil degranulation releases major basic protein, which in turn causes degranulation of mast cells and basophils. An allergic reaction is divided into an immediate response and a late-phase response. The inflammatory response after IgE-mediated mast-cell activation occurs as an immediate reaction, starting within seconds, and a late reaction, which takes up to 8 12 hours to develop. The immediate reaction is due to the activity of histamine, prostaglandins, and other preformed or rapidly synthesized mediators that cause a rapid increase in vascular permeability and the contraction of smooth muscle. The late-phase reaction is caused by the induced synthesis and release of mediators including leukotrienes, chemokines, and cytokines from the activated mast cells. Although the late-phase reaction is clinically less marked than the immediate response, it is associated with a second phase of smooth muscle contraction, sustained edema, and the development of one of the cardinal features of allergic asthma: airway hyperreactivity to nonspecific bronchoconstrictor stimuli such as histamine and methacholine. The late-phase reaction is an important cause of much serious long-term illness, as for example in chronic asthma. The clinical effects of allergic reactions vary according to the site of mast-cell activation. When reexposure to allergen triggers an allergic reaction, the effects are focused on the site at which mast-cell degranulation occurs. In the immediate response, the preformed mediators released are short-lived, and their potent effects on blood vessels and smooth muscles are therefore confined to the vicinity of the activated mast cell. The more sustained effects of the late-phase response are also focused on the site of initial allergen-triggered activation, and the particular anatomy of this site may determine how readily the inflammation can be resolved. Thus, the clinical syndrome produced by an allergic reaction depends critically on three variables: the amount of allergenspecific IgE present; the route by which the allergen is introduced; and the dose of allergen. If an allergen is introduced directly into the bloodstream or is rapidly absorbed from the gut, the connective tissue mast cells associated with all blood vessels can become activated. Disseminated mast-cell activation has a variety of potentially fatal effects: the widespread increase in vascular permeability leads to a catastrophic loss of blood pressure; airways constrict, causing difficulty in breathing; and swelling of the epiglottis can cause suffocation. It can occur if drugs are administered to people who have IgE specific for that drug, or after an insect bite in individuals allergic to insect venom. Some foods, for example peanuts or brazil nuts, can cause systemic anaphylaxis in susceptible individuals. This syndrome can be rapidly fatal but can usually be controlled by the immediate injection of epinephrine, which relaxes the smooth muscle and inhibits the cardiovascular effects of anaphylaxis. The most frequent allergic reactions to drugs occur with penicillin and its relatives. In people with IgE antibodies against penicillin, administration of the drug by injection can cause anaphylaxis and even death.
Assistant Professor Emeritus of Orthopaedic Surgery [2004; 1967] Barbara Young diabetes 2 cure discount januvia master card, M diabetic diet plan januvia 100mg with mastercard. Assistant Professor Emeritus of Orthopaedic Surgery [1992; 1961] Veena Kalmanje Acharya blood glucose 435 order januvia, M gestational diabetes test vancouver order januvia 100 mg amex. Instructor in Plastic and Reconstructive Surgery [2009] Kristin Whitford Baranano, M. Instructor in Anesthesiology and Critical Care Medicine [2011] Karen Beth Bleich, M. Instructor in Anesthesiology and Critical Care Medicine [2009; 2006] Glendaliz Bosques, M. Instructor in Oncology [2006], Joint Appointment in Medicine [2008] Tracee Burroughs, M. Instructor in Otolaryngology-Head and Neck Surgery [2011] Franklin Earl Chatham, M. Instructor in Otolaryngology-Head and Neck Surgery [2011] Brendan Joseph Collins, M. Instructor in Medicine [2011; 2009] (from 09/01/2011), Research Associate in Medicine [2009] (to 08/31/2011) Mary Cheffings Eaton, Ph. Instructor in Plastic and Reconstructive Surgery [2003] Virginia MacVeagh Ferrante, M. Instructor in Psychiatry [1974], Joint Appointment in Medicine [2002] Joan Audrey Freedman, M. Instructor in Molecular and Comparative Pathobiology [2011; 1998] (from 10/01/2011), Research Associate in Molecular and Comparative Pathobiology [1998] (to 09/30/2011) Theresa J. Instructor in Ophthalmology [2011; 2010] (from 09/01/2011) Shanmugasundaram Ganapathy Kanniappan, Ph. Instructor in Radiation Oncology and Molecular Radiation Sciences [2010] Cherilyn C. Instructor in Otolaryngology-Head and Neck Surgery [2011] Arthur McLean Hildreth, M. Instructor in Physical Medicine and Rehabilitation [2004] Avril Melissa Houston, M. Instructor in Anesthesiology and Critical Care Medicine [2010] Ann Elizabeth Jones, M. Instructor in Anesthesiology and Critical Care Medicine [2001] Candice Watters Jones, M. Assistant Professor of Psychiatry [2011; 2010] (from 08/01/2011), Instructor in Psychiatry [2010] (to 07/31/2011) Mitchel A. Instructor in Physical Medicine and Rehabilitation [2010] Richard Sang Un Lee, Ph. Instructor in Oncology [1979], Instructor in Otolaryngology-Head and Neck Surgery [2010] Nikita A. Instructor in Gynecology and Obstetrics [2011] (from 08/01/2011) Robert Fielding Pegues, M. Instructor in Radiation Oncology and Molecular Radiation Sciences [2010], Instructor in Oncology [2010] Adam Craig Reese, M. Instructor in Emergency Medicine [2008] (to 07/31/2011) Jeffrey Lawrence Wexler, M. Assistant (Audiology & Speech) in OtolaryngologyHead and Neck Surgery [1998] Stephen Bowditch, M. Assistant (Audiology & Speech) in OtolaryngologyHead and Neck Surgery [2004] Kristin Marie Ceh, M. Assistant (Audiology & Speech) in OtolaryngologyHead and Neck Surgery [1998] Alan B. Assistant (Audiology & Speech) in OtolaryngologyHead and Neck Surgery [2008] Joel Mark Fradin, M. Assistant (Audiology & Speech) in OtolaryngologyHead and Neck Surgery [2002] Christopher Robert Gilbert, D.
A vast array of monoclonal antibodies have now been produced against a wide variety of human tumor-associated antigens (reviewed in Chapter 20 diabetic zucchini bread splenda generic 100mg januvia overnight delivery. In the generation of most monoclonal antibodies diabetic diet quantity purchase generic januvia online, mice are immunized against a specific antigen blood glucose serum buy cheap januvia on-line, and their cells are fused with the mouse myeloma cell blood sugar sex magik tab cheap januvia. It is possible, however, to use human lymph node or peripheral blood cells for fusion with human myelomas. These human monoclonal antibodies may be able to identify antigens that are not immunogenic in the mouse and are less immunogenic in humans than murine monoclonal antibodies. It is possible to make recombinant chimeric monoclonal antibodies that contain the variable region of murine origin and the constant region of human origin. Virtually all monoclonal antibodies have at least some reactivity with normal tissues, although the degree of cross-reactivity can be minimal. The potential clinical applications of monoclonal antibodies are summarized in Table 18-12 and Chapter 20. Active immunotherapy refers to the immunization of the tumor-bearing host with materials designed to elicit an immune reaction capable of eliminating or retarding tumor growth. These early approaches were almost uniformly unsuccessful in humans and have largely been abandoned. Classification of Cancer Immunotherapies the advent of recombinant cytokines provided a more selective means for stimulating the immune system. Seven percent of patients with melanoma underwent a complete response, and 9% underwent a partial response. In patients with renal cancer the complete and partial response rates were 9% and 10%, respectively. Of 12 patients with metastatic melanoma who achieved a complete response, only two have recurred, with the remainder having ongoing complete responses of between 95 and 173 months. Of 21 patients with metastatic renal cell cancer who achieved a complete regression, four have recurred and 17 have ongoing responses between 46 and 159 months. Complete response to treatment with high-dose interleukin-2, with deaths due to other causes censored. The number of variables involved in determining optimal methods of immunization of humans presents a daunting challenge and are shown in Table 18-17. Variables Involved in the Immunization of Humans against Cancer Antigens A variety of cancer antigens are available. Similarly, these antigens can be presented on professional antigen-presenting cells, such as mature dendritic cells. Because of the myriad of possibilities for immunization of humans, substantial effort has been placed on elucidation in animal models of the general principles for vaccination against cancer antigens. Four general principles resulting from these murine studies are presented in Table 18-18 and have guided the work in humans. General Principles for Human Vaccination against Cancer Antigens Based on Animal Models It is possible to immunize cancer patients against antigens present on their autologous cancers and raise high levels of circulating immune lymphocytes. In most experimental systems, the transfer of immune cells, but not of antibody directed against cellular antigens, produces immunity to tissue transplantations. The major obstacle to the development of successful adoptive immunotherapies for the treatment of cancer in humans has been the inability to develop immune cells with specific reactivity for human tumors that could be obtained in large enough numbers for transfer to tumor-bearing patients. However, several new approaches have been developed for generating human cells with reactivity to tumor. Beginning in 1980, Rosenberg and colleagues 312,313 and 314 described a technique for generating lymphoid cells from mice and humans that were capable of lysing fresh tumor cells but not normal cells. Improved methods for developing lymphocytes with antitumor activity by in vitro sensitization with tumor-specific peptides, as well as the use of cloned cells, are under development and may provide valuable reagents for use in future adoptive immunotherapy studies. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. Molecular cloning and characterization of a second subunit of the interleukin 1 receptor complex.
100 mg januvia visa. Les Fruits Secs Font-ils Grossir ? Raisins secs Abricots Secs Pruneaux....
Syndromes
- Breathing support (artificial respiration)
- Developmental delay
- Two weeks before surgery, your doctor or nurse may ask you to stop taking medicines that make it harder for your blood to clot. These include aspirin, ibuprofen (Advil, Motrin), naproxen (Aleve, Naprosyn).
- Kidney failure
- ECG
- Persistent high fever
- Bilirubin level
- Changes in female body contours
- Falling overboard from a boat into cold water
- ADAMTS 13 activity level
Professor of Radiology [2001; 1982] diabetes in kittens signs buy januvia pills in toronto, Professor of Pathology [2005] diabetes death buy 100mg januvia with mastercard, Professor of Urology [2001; 1997] James Tahara Handa diabetic diet kraft order januvia 100mg with amex, M diabetes mellitus education buy 100mg januvia with amex. Ort Professor of Ophthalmology [2009], Professor of Biomedical Engineering [2009; 1998], Professor of Neurological Surgery [2009], Professor of Oncology [1998; 2005] Daniel F. Jeffrey and Harriet Legum Professor of Acute Neurological Medicine in the Department of Neurology [1996; 1983], Professor of Anesthesiology and Critical Care Medicine [1996; 1983], Professor of Neurological Surgery [1996; 1984] Joshua Michael Hare, M. Adjunct Professor of Medicine [2007; 1995], Adjunct Professor of Biomedical Engineering [2007; 2003] Merel H. Visiting Professor of Anesthesiology and Critical Care Medicine [1987; 1985] John W. Professor of Psychiatry [1997; 1976] (on leave of absence to 12/31/2011), Professor of Pediatrics [1997; 1976] (on leave of absence to 12/31/2011) Gerald Warren Hart, Ph. DeLamar Professor of Biological Chemistry [1997], Director of the Department of Biological Chemistry [1997] Paul M. Professor of Oncology [1991], Professor of Pathology [1997], Professor of Pharmacology and Molecular Sciences [1988; 1976] S. Professor of Oncology [1995; 1991], Associate Professor of Neurology [1989; 1988], Professor of Pharmacology and Molecular Sciences [1995; 1976] David Bruce Hellmann, M. Aliki Perroti Professor of Medicine [1996; 1970], Vice Dean for the Bayview Campus [2005] Lee J. Professor of Oncology [1999], Professor of Pediatrics [1999] Craig Walter Hendrix, M. Professor of Medicine [2009; 1994], Professor of Pharmacology and Molecular Sciences [2009; 1999] Stewart H. Adjunct Professor of Behavioral Biology in the Department of Psychiatry [2002; 1992] James Gordon Herman, M. Professor of Neurology [2006; 1999], Professor of Physical Medicine and Rehabilitation [2006; 2004] Charles W. Professor of Anesthesiology and Critical Care Medicine [2010; 2005] Ahmet Hoke, M. Professor of Pathology [1999; 1990], Professor of Oncology [1999; 1995] Steven Shih-ting Hsiao, Ph. Professor of Neuroscience [2008; 1992], Professor of Biomedical Engineering [2008; 1999] Shau-Ku Huang, Ph. Professor of Cell Biology [1986; 1980], Joint Appointment in Medicine [1987], Professor of Physiology [1993] Richard L. Professor of Neuroscience [1993; 1988], Joint Appointment in Biological Chemistry [1989], Director of the Department of Neuroscience [2006], Investigator of the Howard Hughes Medical Institute Thierry A. Adjunct Professor of Behavioral Biology in the Department of Psychiatry [1997] Nicholas Taylor Iliff, M. William Thomas Gerrard, Mario Anthony Duhon and Jennifer and John Chalsty Professor of Urology [1998; 1988], Professor of Oncology [1998; 1992] Ethylin Wang Jabs, M. Adjunct Professor of Pediatrics [2007; 1984], Adjunct Professor of Medicine [2007; 1990], Adjunct Professor of Plastic and Reconstructive Surgery [2007; 1984] David L. Baxley Professor of Pathology [1996], Director of the Department of Pathology [2001] Elizabeth M. Dana and Albert "Cubby" Broccoli Professor of Oncology [2002; 1992], Professor of Pathology [1999] George Issa Jallo, M. Professor of Neurological Surgery [2010; 2003], Professor of Oncology [2010; 2003], Professor of Pediatrics [2010; 2003] Kay Redfield Jamison, Ph. Dalio Family Professor of Mood Disorders in the Department of Psychiatry [1993; 1987] Henry D. Professor of Cell Biology [2004; 1988], Professor of Biological Chemistry [2010] Roger Anthony Johns, M. Professor of Anesthesiology and Critical Care Medicine [1999], Professor of Medicine [2006], Director of the Department of Anesthesiology and Critical Care Medicine [2003; 1999] Richard T. University Distinguished Service Professor of Neurology [1969], Professor of Neuroscience [1983], Director of the Department of Neurology [1997; 1988] Rolley E. Professor of Neurology [1988], Professor of Pediatrics [1988], Professor of Physical Medicine and Rehabilitation [2005] Bronwyn Jones, M. Adjunct Professor of Behavioral Biology in the Department of Psychiatry [2010; 1998] Richard J.