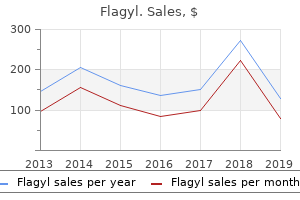
"Purchase 500 mg flagyl overnight delivery, antibiotic resistant urinary infection".
By: L. Taklar, MD
Program Director, Albany Medical College
A good napping lubricant antibiotic resistant bronchitis buy flagyl with paypal, for example infection urinaire buy cheap flagyl 250mg online, provides lubrication between the fabric and the napping wires yet a t the same time provides a certain amount of cohesiveness between fibers antibiotic resistance animal agriculture purchase 200mg flagyl mastercard. Sulfonated oils (eg Turkey Red Oil) impart a soft raggy hand oral antibiotics for acne effectiveness order flagyl 250 mg on-line, sulfonated tallow a full waxy hand and sulfonated fatty esters a smooth waxy hand. Advantages Most anionic softeners show good stability towards heat and some are resistant to yellowing. Anionic softeners do not interfere with finishes to be foamed, in fact 142 like defoamers and are deleterious for foam finishing. Anionic softeners have good rewetting properties and are preferred for those fabrics t h a t must adsorb water such a s bath towels. Disadvantages the degree of softness with anionics is inferior when compared with cationics and some nonionics. Generally speaking, more anionic product must be used and even then, the cationics and some nonionics impart a softer, fluffier feel to the fabrics. Anionics will not exhaust from a bath, they must be physically deposited on the fabric. Anionics tend to be sensitive to water hardness a n d to electrolytes in finish baths. Anionics are incompatible in some finish baths containing cationically stabilized emulsions. Cationic Softeners Cationic softeners are ionic molecules t h a t have a positive charge on the large part of the molecule. The important ones a r e based on nitrogen, either in the form of a n amine or in the form of a quaternary ammonium salt. The amine becomes positively charged a t acidic pHs and therefore functions as a cationic material at pH below 7. An important quality of cationic softeners is that they exhaust from water onto all fibers. When in water, fibers develop a negative surface charge, setting up a n electronic field for attracting positively charged species. These forces causes the cationic softener to deposit i n a n oriented fashion, the positive end of the softener molecule is attracted to the fiber surface forcing the hydrocarbon tail to orient outward. The fiber now takes on low energy, nonpolar characteristics; therefore, the fiber has the lowest possible coefficient of friction. The ionic attraction causes complete exhaustion from baths and the orientation on the fiber surfaces allows a monolayer to-be as effective as having more lubricant piled on-top. Amine Functional Cationic Softeners Long chain amines are not water soluble a t neutral a n d alkaline pH; however, when converted to their acid salt, they develop a cationic charge and become water soluble. The cationic charge on a given hydrophobe is proportional to the number of amino groups, therefore the attraction of the cationic protion to the fiber surface increases a s the number of amine groups increase. These intermediates can function either as softeners or be used to make other derivatives. A second method of making aminofunctional molecules is to make aminoesters or animoamides of fatty acids. Cationic Amine Salts Fatty amines derived from tallow fatty acids are called tallow or di-tallow amines, those made from coconut acids would be called coco amines or di-coco amines. Fatty Aminoesters Aminoesters are made by reacting alkanol amines with fatty acids. These materials too become cationic under acidic conditions, the strength of the cationic 144 charge is proportional to the number of amino groups. Examples of alkanol amines are ethanol amine, diethanol amine and hydroxyethyl-ethylene diamine. Fatty Amidoamides Aminoamides are made by the condensation of polyamines with fatty acids. Ethylene diamine, N,N-diethylethylene diamine and diethylene triamine are examples of polyamines that a r e condensed with fatty acids. Usually, the fatty acids a r e commercial grades such as would be derived from tallow or coconut oil. The products would then carry a generic name such a s tallow aminoamides or coco aminoamides. Acetic acid, hydrochloric acid, sulfuric acid and citric acid salts of many of them are commercially available for use a s softeners.
Two weak bonds that occur frequently are hydrogen bonds and van der Waals interactions virus x-terminator order generic flagyl from india. Because the hydrogen is slightly positive first line antibiotics for acne flagyl 500mg with mastercard, it will be attracted to neighboring negative charges antibiotics kidney cheap flagyl 400 mg online. This type of bond is common 46 Chapter 2 the Chemical Foundation of Life and occurs regularly between water molecules antibiotic resistance epidemic 200 mg flagyl overnight delivery. Individual hydrogen bonds are weak and easily broken; however, they occur in very large numbers in water and in organic polymers, creating a major force in combination. Like hydrogen bonds, van der Waals interactions are weak attractions or interactions between molecules. Van der Waals attractions can occur between any two or more molecules and are dependent on slight fluctuations of the electron densities, which are not always symmetrical around an atom. For these attractions to happen, the molecules need to be very close to one another. These bonds-along with ionic, covalent, and hydrogen bonds-contribute to the three-dimensional structure of the proteins in our cells that is necessary for their proper function. Pharmaceutical Chemist Pharmaceutical chemists are responsible for the development of new drugs and trying to determine the mode of action of both old and new drugs. Drugs can be found in the natural environment or can be synthesized in the laboratory. In many cases, potential drugs found in nature are changed chemically in the laboratory to make them safer and more effective, and sometimes synthetic versions of drugs substitute for the version found in nature. After the initial discovery or synthesis of a drug, the chemist then develops the drug, perhaps chemically altering it, testing it to see if the drug is toxic, and then designing methods for efficient large-scale production. This process often takes several years and requires the participation of physicians and scientists, in addition to chemists, to complete testing and gain approval. An example of a drug that was originally discovered in a living organism is Paclitaxel (Taxol), an anti-cancer drug used to treat breast cancer. Finding drugs often means testing hundreds of samples of plants, fungi, and other forms of life to see if any biologically active compounds are found within them. Sometimes, traditional medicine can give modern medicine clues to where an active compound can be found. For example, the use of willow bark to make medicine has been known for thousands of years, dating back to ancient Egypt. It was not until the late 1800s, however, that the aspirin molecule, known as acetylsalicylic acid, was purified and marketed for human use. Occasionally, drugs developed for one use are found to have unforeseen effects that allow these drugs to be used in other, unrelated ways. For example, the drug minoxidil (Rogaine) was originally developed to treat high blood pressure. When tested on humans, it was noticed that individuals taking the drug would grow new hair. Eventually the drug was marketed to men and women with baldness to restore lost hair. The career of the pharmaceutical chemist may involve detective work, experimentation, and drug development, all with the goal of making human beings healthier. Water is one of the more abundant molecules and the one most critical to life on Earth. The polarity of the water molecule and its resulting hydrogen bonding make water a unique substance with special properties that are intimately tied to the processes of life. Special properties of water are its high heat capacity and heat of vaporization, its ability to dissolve polar molecules, its cohesive and adhesive properties, and its dissociation into ions that leads to the generation of pH. Understanding these characteristics of water helps to elucidate its importance in maintaining life. A polar substance that interacts readily with or dissolves in water is referred to as hydrophilic (hydro- = "water"; -philic = "loving"). In contrast, non-polar molecules such as oils and fats do not interact well with water, as shown in Figure 2. These nonpolar compounds are called hydrophobic (hydro- = "water"; -phobic = "fearing").
Purchase flagyl 250 mg with visa. 6 Amazing Herbs That Lower Blood Sugar Naturally.
We will refer back to this table frequently as we discuss the basic chemistry of the elements antibiotics used for cellulitis cheap flagyl 500mg amex. The 9 elements highlighted in green virus structure order flagyl 400mg visa, along with those in yellow antibiotic resistant virus in hospitals cheap flagyl 200mg otc, are considered major essential elements virus scanner purchase flagyl 250 mg on-line. The elements highlighted in blue are considered minor essential elements and are required only in trace amounts in the body. Often these symbols are the first letter or letters in the name of the element; H for hydrogen, C for carbon, and He for helium. Occasionally, however, the symbols represent the Latin name for the element, hence the symbol for sodium is Na for the Latin Natrium and the symbol for Potassium is K for the Latin Kalium. Of the 92 naturally occurring elements, 4 make up roughly 96% of our body weight, namely Carbon (C), Hydrogen (H), Oxygen (O) and Nitrogen (N) (Figure above, yellow highlight). In addition to these four, there are a number of other important, 20 but less abundant, elements found in the body. These include phosphorus, sodium, potassium, calcium, magnesium, sulfur, chlorine, iron, and iodine (Figure above, green highlight). Several other elements are also required for normal functioning but only in trace amounts (Figure above, blue highlight). We can define an atom as the simplest particle of an element that has the chemical properties of that element. Chemical properties include the physical state of the element (gas, liquid, or solid), the types of bonds the element can form, how it reacts with other elements, etc. Therefore all of the atoms that make up the element carbon have the same chemical properties. Physicists have succeeded in blasting atoms apart into dozens of different subatomic particles, however, only 3 of them are stable. Protons are positively charged particles, have mass, and are located in the center, or nucleus of the atom. Neutrons have no charge, have mass, and are also located in the nucleus of the atom. Too many or too few neutrons may result in an atomic nucleus that is unstable and may decay to form other elements. Although the mass of the neutron is slightly greater than that of a proton we can assign both of them the relative mass of 1 (1 atomic mass unit or amu). Electrons have a negative charge but are extremely small and have a mass only 1/1850 that of a proton or neutron. They are so small that for practical purposes they do not contribute to the mass of the atom. Electrons move around the nucleus at tremendously high speeds, actually travelling at near the speed of light. Although we often describe the electrons as residing in orbits that circle the nucleus, like planets orbiting the sun, modern physics teaches us that this model is incorrect. These "orbitals" are actually areas in space around the nucleus where the electrons will be located most of the time. For simplicity, however, we often think of these as satellite-like circular orbitals. This number is the atomic number for the element and is unique for each different element. For carbon, the atomic number is 6 and, again, no other element has an atomic number of 6. The significance of the atomic 22 number is that it tells us the number of protons in the nucleus of each element. In addition, since atoms have a neutral charge, the atomic number also tells us the number of electrons in the atom. In chemical notation the atomic number for an element is expressed as a subscript preceding the symbol for the element. Mass Number (Atomic Mass) the mass number of an atom, as the name implies, tells the total mass of the atom. Also, recall that the mass of each proton as well as each neutron is 1 atomic mass unit. Since the mass number is the number of protons plus the number of neutrons and the atomic number is the number of protons, you can find the number of neutrons by simply subtracting the atomic number from the mass number.
Treatment of D-lactic acidosis involves the administration of antibiotics to decrease the bacterial synthesis of D-lactate antibiotics for uti late period cheap flagyl 200mg with mastercard, as well as special diets low in carbohydrates bacteria unicellular or multicellular cheap flagyl amex. Topf 13 Metabolic Acidosis: Anion Gap Ketoacidosis Ketoacidosis occurs when the body is unable to use glucose as an energy source antibiotics for sinus infection diarrhea generic 200mg flagyl amex. Glucose is normally the primary fuel antibiotic resistance patterns flagyl 200mg low cost, but ketones become the primary energy source when glucose cannot be used. In order to understand ketoacidosis, it is important to understand how the body determines its primary fuel source: glucose in the fed state and ketones in the fasting state. Ketoacidosis occurs in three different clinical scenarios:. diabetes and ingestion. In response, the pancreas secretes insulin and shuts off the secretion of glucagon. This is known as the fed state during which the primary fuel for the body is glucose. Insulin promotes the building of muscle, storage of fat and hepatic glycogen synthesis. Topf 13 Metabolic Acidosis: Anion Gap Ketoacidosis In the fasting state, glucagon predominates and ketones are the primary fuel source. During fasting, as blood sugar falls, the pancreas releases glucagon and suppresses insulin. Initially, the liver converts glycogen stores into glucose to prevent hypoglycemia. If the fasting state is prolonged, the primary fuel is switched from glucose to ketones. Glucagon stimulates the liver to convert glycogen to glucose, fatty acids to ketones and alanine to glucose. These hormones (the counter-regulatory hormones) promote lipolysis, protein catabolism and gluconeogenesis. Norepinephrine is responsible for the symptoms of hypoglycemia: sweating, tremors and agitation. Glucagon acts in the to increase the conversion of amino acids and glycogen into and the conversion of fatty acids into. Decreased insulin stimulates the breakdown of triglycerides into free fatty acids, the substrates for ketone formation. Ketosis is normally self-limited because an increase in plasma ketones triggers the release of insulin which suppresses ketogenesis. Ketoacidosis only becomes clinically significant when insulin activity is blocked. The initial response to a fast is the breakdown of stored glycogen to glucose (glycogenolysis) in the liver. Glycogen supplies are limited, however, and are typically exhausted within 24 hours. The next response to a prolonged fast is the catabolism of protein into alanine and then into glucose via gluconeogenesis. In order to minimize the loss of essential muscle mass, the primary energy source in long-term starvation is the catabolism of fat. Adipocytes release triglycerides which are hydrolyzed into glycerol and fatty acids. Glucose can be produced from glycerol, but the majority of energy from triglycerides is derived from the fatty acids. Normally, the presence of ketones stimulates the secretion of insulin, but during starvation insulin secretion is blocked by persistent hypoglycemia. Suppression of gluconeogenesis causes hypoglycemia which prevents insulin secretion.